DEMO TEST n.3
Question 1 / 8
The two non-metals in the following group are:
Question 2 / 8
When marble pieces are added to hydrochloric acid, the following reaction takes place:
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g)Which of the following actions would increase the rate of formation of carbon dioxide?
Question 3 / 8
Compounds that have high melting points, conduct electricity in the molten phase and are often soluble in water are
Question 4 / 8
From the list, select the gases that can be collected using the apparatus shown in the diagram on the left.
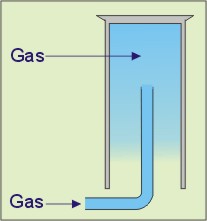
Question 5 / 8
Oxides which produce alkaline solutions when mixed with water include:
Question 6 / 8
Calculate how many moles of ethanoic acid (CH3COOH: relative molecular mass 60) may be produced from 92 g of ethanol (CH3CH2OH: relative molecular mass 46). Answer:
Question 7 / 8
The reaction between zinc (Zn) and hydrochloric acid produces...
Question 8 / 8
Which of the following would yield the greatest amount of useful energy ?